The Use of Catalysts for Hydrogen Fuel Cells
The diagram below shows the structure of a hydrogen fuel cell. The simple working principle of hydrogen fuel cell is as follows: hydrogen gas is sent to the surface of the anode plate (negative electrode) of the fuel cell, and after the catalytic effect of the catalyst, hydrogen decomposes into hydrogen ions and electrons, and the hydrogen ions (proton H+) pass through the proton exchange membrane (PEM) and reach the cathode plate (positive electrode) of the fuel cell. The electrons cannot pass through the PEM directly, but can only reach the fuel cell cathode plate through an external circuit, thus generating a circuit current. After reaching the fuel cell cathode plate, the electrons react with oxygen and hydrogen ions to produce water.
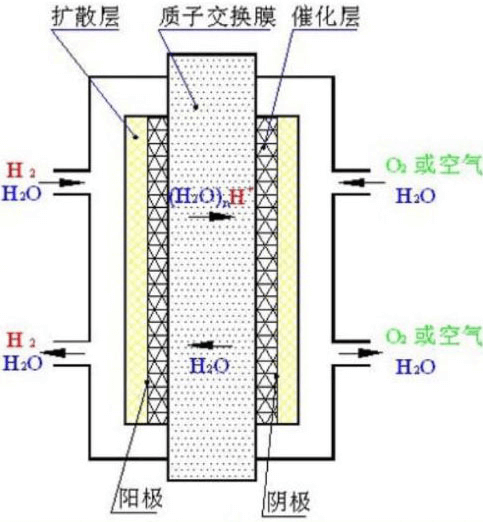
The hydrogen fuel cell is fueled by hydrogen and oxygen, and the only product produced is water. The hydrogen fuel cell does not produce carbon monoxide or carbon dioxide, and there are no pollutants such as sulfur or particulates. Therefore, hydrogen fuel cell car is an electric car with zero emission and zero pollution in the real sense, and hydrogen will be the perfect energy fuel for cars!
Catalyst for the Application on Graphene Hydrogen Fuel Cells
The membrane electrode assembly (MEA), consisting of a proton exchange membrane, catalyst, and gas diffusion layer, is the "heart" of the fuel cell stack (figure below).
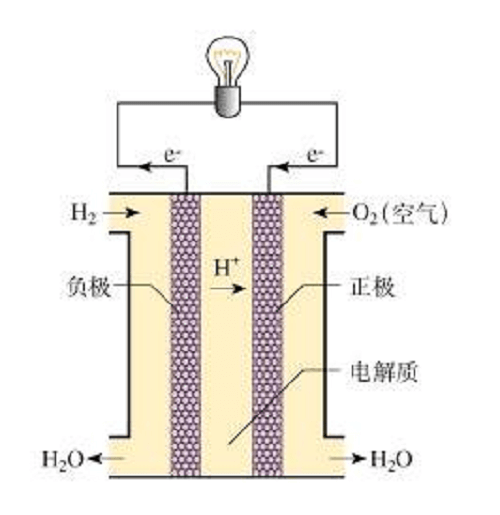
Among them, the catalyst determines the discharge performance and lifetime of the hydrogen fuel cell. Platinum is one of the most commonly used catalysts for fuel cells, as it effectively enables the redox reaction at the center of the technology. Its high cost has stimulated research efforts to find suitable alternatives by looking for ways to use more likely small amounts while maintaining the same catalytic activity. Toyota reduced the platinum loading of Mirai's electric stack to about 0.17 g/kW by optimizing the platinum/cobalt alloy ratio. Honda Clarity's reactor has a low platinum load of 0.12 g/kW due to the use of nitrogen atomic layer technology, but platinum catalysts also suffer from low activity, poor durability, and easy poisoning. Therefore, there is an urgent need to develop new cost-effective catalysts.
Graphene has become a hot topic in hydrogen fuel cell catalyst research because of its high electrical conductivity, large specific surface area, and excellent chemical stability. Graphene doping increases the surface catalytic sites and can be used as a metal-free catalyst itself; graphene surface modification can increase the anchoring sites of loaded metal nanoparticles and is a good carrier for non-platinum metal catalysts.
CARBON SUPPORTED PLATINUM CATALYST FOR FUEL CELLS,PT/C
For example, the topic of successful use of graphene as a replacement for platinum catalysts was investigated. Graphene with a three-dimensional structure was firstly fabricated from graphene, and then the three-dimensional structure was coated with nitrogen and sulfur elements by vapor deposition (CVD). The experimental results showed that the higher the amount of nitrogen and sulfur plated on the graphene surface, the higher the catalytic efficiency for hydrogen and the more efficient the catalytic production of more hydrogen. Based on this research, scientists have found that if nickel nanoparticles are loaded on the surface of graphene catalysts, the catalytic efficiency of hydrogen production can surpass that of conventional platinum-based catalysts. This technology, if scaled up for industrial production and marketed, could significantly reduce the cost of hydrogen fuel cells.
James Tour, a professor at Rice University, has created a highly efficient and durable catalyst for hydrogen fuel cells by attaching ruthenium nanoparticles to the surface of graphene. Experimental test results show that the catalytic performance of the ruthenium-graphene catalyst is comparable to that of a conventional platinum-based alloy.
Follow UIV Chem to find more info, and we welcome your inquiry.





