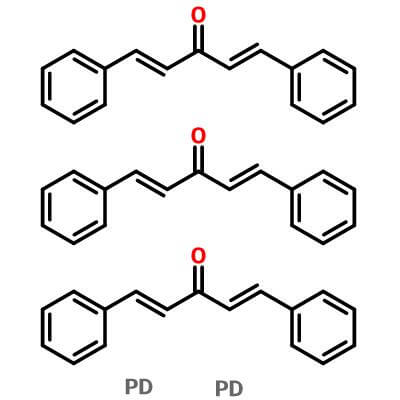
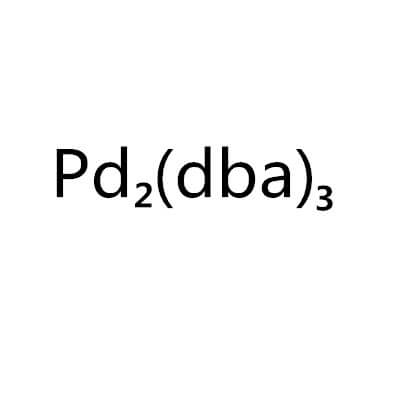Tris(Dibenzylideneacetone)Dipalladium,51364-51-3(52409-22-0;60748-47-2) ,C51H42O3Pd2
| Identification | ||
| Name |
|
Tris(dibenzylideneacetone)dipalladium |
| Synonym |
|
Pd2(dibenzylideneacetone)3
Pd2(dba)3 (C6H5CH=CHCOCH=CHC6H5)3Pd2 |
|
|
||
| Molecular Structure |
|
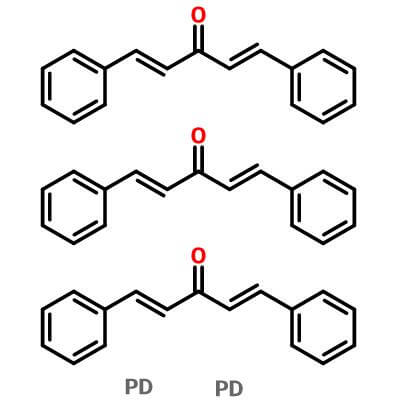 |
|
|
||
| Molecular Formula |
|
C51H42O3Pd2 |
| Molecular Weight |
|
915.73 |
| CAS Registry Number |
|
51364-51-3(52409-22-0;60748-47-2) |
| Pd content |
|
23.2% |
| Specification |
|
Analytical pure |
| Properties | ||
| Melting point |
|
152-155 ºC |
| Water solubility |
|
insoluble;sensitive of air and moisture |
| Appearance |
|
Purple black powder |
| Safety Data | ||
|
Hazard Symbols |
|
Xn |
|
Risk Codes |
|
R20/22 |
| Safety Description |
|
S24/25 |
Tris(dibenzylacetone)dipalladium use:
Tris(dibenzylacetone)dipalladium can be used as a catalyst in the coupling reaction of Suzuki, Kumada, Negishi, Buchwald and other organic synthesis processes in the laboratory.
Tris(dibenzyl acetone)dipalladium is an important zero-valent palladium catalyst, which is widely used in coupling, hydrogenation, carbonylation and other reactions in organic synthesis. In combination with Chemicalbook ligands, in situ formation of high catalytic activity of zero-valent palladium active substances, widely used in carbon-carbon bond formation, carbon-heteroatomic bond formation reactions.
Suzuki coupling reaction catalyst for aryl chloride; Heck coupling reaction catalyst of aryl chloride; Ketone arylation reaction catalyst; Buchwald-Hartwig amino reaction catalyst for aryl halides; Allyl chloride fluoride reaction catalyst; Carboxylate β-aryl reaction catalyst; 1, 1-dichloro-1-olefin carbonylation catalyst; Catalyst for conversion of aryl and vinyl trifluoromethesulfonates to aryl and vinyl halides.
Preparation of tris(dibenzylacetone)dipalladium:
Step one, under a nitrogen atmosphere, in the water bath heating glass reaction kettle with anhydrous ethanol 32 l, 0.85 kg, anhydrous sodium acetate system to join the second when heated to a temperature of 80 ℃ benzylidene acetone 3 kg, mixing reaction after 20 min to add ice water bath heater, cooling the system quickly to 45 ℃, then add 1 kg implementation example 1 of 2 palladium chloride, Bis (dibenzylidene acetone) palladium (0) was obtained by funnel filtration after reaction at 45℃ for 3h. Step 2: Add 5L acetone to the glass reactor heated in a water bath in the atmosphere of nitrogen. When the temperature of acetone in the reactor is heated to 30℃, add double (dibenzyl acetone) palladium (0) obtained in Step 1. React at 30℃ for 3h, filter, wash with deionized water and anhydrous ethanol, dry in the oven at 40℃. The product is tris (dibenzylacetone) dipalladium (0) with purity of 99.8% and yield of 97.3%.
Tris (dibenzylacetone) dipalladium recovery method:
The reduction such as amine with formaldehyde and sodium formate, without first washing and drying of catalysts such as processing, direct solutions to join, oxidation reduction to the metal palladium palladium, the active component with high dispersion particles uniformly distributed on the surface of the carrier, so form a thin layer of palladium on activated carbon, firmly adhere to the outer surface, the unique catalytic activity, Wash it several times with experimental solvents or ethanol, and you'll need more bubbles.