Platinum(II) chloride (PtCl₂,CAS:10025-65-7) is a highly versatile inorganic compound that has gained significant attention in the fields of chemistry, industry, and medicine. With its unique chemical properties, PtCl₂ serves as a foundational material for the synthesis of platinum-based complexes, catalysts, and even life-saving cancer drugs.
What is Platinum(II) Chloride (PtCl₂)?
Platinum(II) chloride is a chemical compound consisting of platinum and chlorine, represented by the formula PtCl₂. It appears as a dark brown to black crystalline powder and is slightly soluble in water. This compound is a cornerstone of platinum coordination chemistry and is widely used in catalysis, materials science, and pharmaceuticals.
Key Properties of PtCl₂
· Chemical Formula: PtCl₂
· Molecular Weight: 265.99 g/mol
· Appearance: Dark brown to black powder
· Solubility: Slightly soluble in water; dissolves in hydrochloric acid to form complex ions like [PtCl4]2−[PtCl₄]^{2-}[PtCl4]2−.
· Structure: PtCl₂ is polymeric in the solid state, with each platinum atom bonded to four chlorine atoms in a square planar geometry.
Applications of Platinum(II) Chloride
It is a multi-functional compound with applications across various fields, including catalysis, coordination chemistry, and medicinal research. Below, we break down its primary uses:
1. Medicinal Chemistry: Key Ingredient in Cancer Drugs
One of the most significant applications of PtCl₂ is in the synthesis of platinum-based anticancer drugs. For example, PtCl₂ is a precursor in the production of cisplatin ([PtCl2(NH3)2][PtCl₂(NH₃)₂][PtCl2(NH3)2]), one of the most widely used chemotherapy drugs for treating cancers such as testicular, ovarian, and lung cancer.
Cisplatin works by binding to the DNA of cancer cells, preventing their replication and ultimately leading to cell death. The success of cisplatin has made PtCl₂ a cornerstone in medicinal chemistry.Reaction Example:
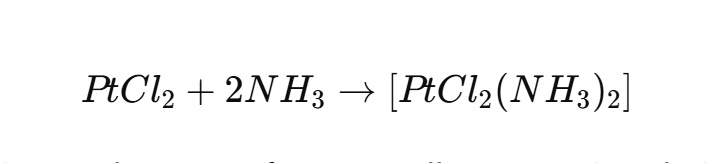
2. Catalysis: A Versatile Catalyst Precursor
Platinum(II) chloride is widely used in organic synthesis as a precursor for catalytic systems. It facilitates key reactions such as hydrogenation, oxidation, and coupling reactions. Its catalytic properties make it invaluable in:
· Pharmaceutical synthesis: Production of active pharmaceutical ingredients (APIs).
· Polymer chemistry: Catalyzing polymerization reactions.
· Fine chemicals: Enabling complex organic transformations in chemical manufacturing.
For instance, PtCl₂ can be used as a catalyst in the addition of hydrogen to alkenes, leading to efficient hydrogenation reactions.
3. Coordination Chemistry: Building Complexes
PtCl₂ is an essential starting material in the preparation of platinum-based coordination compounds. When dissolved in concentrated hydrochloric acid, PtCl₂ forms complex ions such as [PtCl4]2−[PtCl₄]^{2-}[PtCl4]2−, which are used in further chemical synthesis. This ability to form stable complexes makes PtCl₂ a valuable tool in research.
4. Materials Science and Electronics
In the field of materials science, PtCl₂ serves as a precursor for synthesizing platinum-based materials, including conductive films and catalysts for fuel cells. Its unique properties, such as chemical stability and conductivity, make it suitable for high-performance electronic components and sensors.
How to Buy High-Quality Platinum(II) Chloride
When sourcing PtCl₂, it’s important to consider the following factors:
1. Purity: Laboratory-grade PtCl₂ (≥99%) is recommended for research and industrial applications.
2. Supplier Reputation: Ensure you purchase from trusted chemical suppliers to guarantee consistent quality.
3. Storage Conditions: PtCl₂ should be stored in a dry, sealed container to prevent contamination or degradation.
Why is Platinum(II) Chloride Important?
Platinum(II) chloride plays a critical role in modern science and technology. From enabling life-saving cancer treatments to serving as a catalyst in chemical synthesis, its versatility is unmatched. As research into platinum-based compounds continues, PtCl₂ will undoubtedly remain a key player in advancing medical, industrial, and technological innovation.
Conclusion
Platinum(II) chloride (PtCl₂,CAS:10025-65-7) is far more than just an inorganic compound. Its unique chemical properties and wide-ranging applications make it indispensable in fields such as medicine, catalysis, and materials science. Whether you’re a researcher, chemist, or industrial professional, PtCl₂ is a compound that holds immense potential.
If you’re looking to purchase platinum(II) chloride or learn more about its uses, this guide provides a comprehensive overview. Bookmark this page for future reference, and explore the incredible versatility of PtCl₂ in chemistry and beyond!





