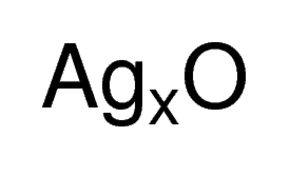Silver Oxide,20667-12-3,Ag2O,Silver(I) Oxide
| Identification | |||
| Name |
|
Silver oxide | |
| Synonyms |
|
Silver(I) oxide SILVER OXIDE pure Oxydisilver(I) |
|
| Molecular Formula |
|
Ag2O | |
| Molecular Weight |
|
231.74 | |
| CAS Registry Number |
|
20667-12-3 | |
| EINECS |
|
243-957-1 | |
| No. |
Item |
Specification(Cell Grade) |
Result |
|
| 1 |
Ag2O |
≥99.5 (wt%) |
99.52% |
|
| 2 |
Loss on drying(120℃) |
≤0.250 (wt%) |
0.23 |
|
| 3 |
Free base (NaOH) |
≤0.012 (wt%) |
0.010 |
|
| 4 |
Hydrochloric acid does not precipitate |
≤0.10 (wt%) |
0.060 |
|
| 5 |
Cu |
≤0.003 (wt%) |
0.028 |
|
| 6 |
Fe |
≤0.003 (wt%) |
0.025 |
|
| 7 |
Nitrate(NO3) |
≤0.05 (wt%) |
0.045 |
|
| 8 |
Nitrate(in CO2 terms) |
≤0.1 (wt%) |
0.082 |
|
| 9 |
Surface density (g/cm3) |
0.85-1.2 |
0.93 |
|
| 10 |
Impact Density(g/cm3) |
1.4-2.0 |
1.55 |
|
| 11 |
Particle size distribution: |
<1um |
<10% |
8% |
|
<1-10um |
80-100% |
90% |
||
|
<10um |
<10% |
2% |
||
| Properties | |||
| Water solubility |
|
slightly soluble | |
|
Melting point |
|
300°C |
|
|
Density |
|
7,143 g/cm3 |
|
| Safety Data | |||
| Hazard Symbols |
|
O;C | |
| Risk Codes |
|
R34;R8 | |
| Safety Description |
|
S17;S26;S36/37/39;S45 | |
| Transport Information |
|
UN 1479 | |
Silver Oxide uses:
Used in pharmacy and organic synthesis, as glass polishing agent, coloring agent, battery plate, catalyst and water purifier.
Used as oxidant, analytical reagent and preservative.
Used in medical treatment and as glass polishing agent, coloring agent and water purifying agent.
Mainly used as a chemical synthesis catalyst. It is also used as a preservative, electronic device material, glass colorant and abrasive.
Silver oxide is the electrode material for silver oxide batteries. It is also a weak oxidant and weak base in organic synthesis. It can react with 1,3-disubstituted imidazole salts and benzimidazole salts to form nitrogen heterocyclic carbenes, which can replace unstable ligands cyclooctadiene or acetonitrile as carbenes. Transfer reagents to synthesize transition metal carbene complexes. In addition, silver oxide can convert organic bromides and chlorides into alcohols, and benzyl halides into benzyl ethers under low temperature and the presence of water vapor. It can be used in combination with methyl iodide as a methylating reagent for sugars. Methylation analysis and Hoffman elimination reaction, as well as oxidation of aldehydes to carboxylic acids. It is also used as a surface catalyst for the epoxidation of alkenes.
Silver Oxide production method:
The silver nitrate method reacts the silver nitrate solution with the sodium hydroxide solution, and the generated silver oxide is washed, separated, and dried to obtain a finished product. The reaction formula is: 2AgNO3+2NaOH→Ag2O+2NANO3+H2O