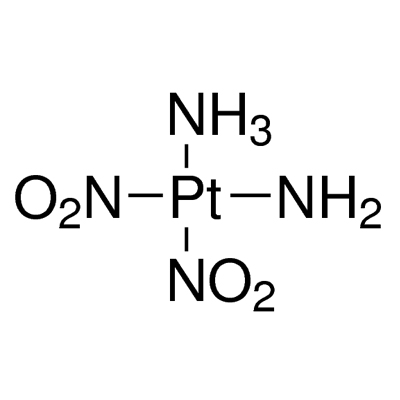
Diammineplatinum(II) Nitrite,14286-02-3,Pt(NH3)2(NO2)2
| Identification | ||
| Name |
|
Diamminedinitritoplatinum(II) |
| Synonyms |
|
Diammineplatinum(II) nitrite diamminebis(nitrito-n)-platinu Diammineplatinum(II) nitrite solution Diammineplatinum dinitrite Dinitrodiammine platinum |
|
|
||
| Molecular Structure |
|
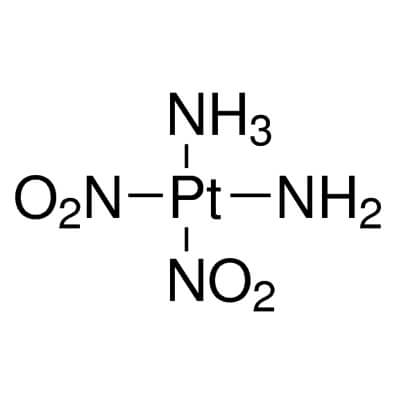 |
|
|
||
| Molecular Formula |
|
Pt.(NH3)2.(NO2)2 |
| Molecular Weight |
|
321.15 |
| CAS Registry Number |
|
14286-02-3 |
| EINECS |
|
238-203-3 |
| Properties | ||
|
Density |
|
1.015 g/mL at 25 °C |
| Platinum content |
|
59%up |
| Purity |
|
Purity of original platinum powder>99.95% |
| Appearance |
|
White crystal |
| Property |
|
White crystal, insoluble in cold water, soluble in cold water, ammonia. |
| Application |
|
Common material for platinizing liquor; used in preparing catalyst |
| Specification |
|
Analytical pure |
| Safety Data | ||
| Hazard Symbols |
|
Xi,N |
|
Risk Codes |
|
42/43-46-61-50-36/37/38 |
|
Safety Description |
|
23-36-43-45-61-26 |
|
Dangerous Goods Transport Number |
|
2810 |
As a global manufacturer and exporter of high-performance platinum-based chemicals, UIV Chem offers premium-grade Diamminedinitritoplatinum (CAS No. 14286-02-3) tailored for advanced pharmaceutical, catalytic, and R&D applications. All products are proudly made in China and supported by full export documentation and technical validation.
Diammineplatinum(II) Nitrite Key Features
High Purity & Batch Consistency: Manufactured in ISO-compliant facilities with strict quality assurance protocols.
Global Export Compliance: Full documentation including COA, MSDS, Reach/TSCA support for hassle-free customs clearance.
Reliable Supply Chain: Stable inventory and responsive lead times for both trial and large-scale orders.
Expert Technical Support: Our experienced chemists offer fast, professional support for formulation, documentation, and logistics.
Diamminedinitritoplatinum(II) application:
Dinitrosodiammine platinum [Pt(NH3)2(NO2)2], commonly known as P salt, is mainly used for platinum plating in non-cyanide electroplating. The resulting coating has high hardness and low resistance, and can be welded; the surface of electronic components is plated with platinum .
Diamminedinitritoplatinum(II) uses:
Used in the electroplating industry
Electroplating reagents:
1. Commonly used reagents for platinum plating in cyanide-free electroplating, the resulting coating has high hardness, low resistance, and can be welded;
2. Used for platinum plating on the surface of electronic components;
3. Corresponding P salt crystals can be obtained by crystallization of the product.
Diamminedinitritoplatinum (II) preparation:
Weigh 195g of metallic platinum, wash it and put it into a beaker, add an appropriate amount of aqua regia, heat it appropriately to start the reaction, and it is better to make the solution bubble evenly for the reaction. The reaction was stopped by adding hydrochloric acid to drive nitrate until brown gas no longer came out, then transferred to a rotary evaporator, concentrated to anhydrous and steamed out, transferred to a beaker and added an appropriate amount of water to prepare a chloroplatinic acid solution with a concentration of 200g/L. Weigh 52.5 g of hydrazine hydrochloride, prepare a 35% deionized aqueous solution, and add it dropwise to the above chloroplatinic acid solution to obtain a scarlet chloroplatinic acid solution. After heating the chloroplatinic acid solution to boiling, add boiling 10 times the molar amount of ammonia, heating until the final solution is colorless, and evaporate and concentrate to precipitate 345.2 g of white dichlorotetraammine platinum. Add 3L of hot deionized water, and then add a saturated solution containing 204g of sodium nitrite. The mixture is heated on a water bath until the yellow precipitate turns into colorless, cooled and crystallized, filtered and recrystallized with hot water to obtain 256g of P salt, with a yield of 80.1 %. Dinitrosodiammine platinum (P salt) [Pt(NH3)2(NO2)2] prepared by this method was tested by ICP, the platinum content was 59.7±0.2%, the sodium ion was 15ppm, the potassium ion was not detected, and the chlorine The ion is 10ppm, and the total content of other metal ions does not exceed 50ppm.
Why Choose UIV Chem
Over 15 Years of Export Experience: Serving clients across North America, Europe, and Asia.
Strict Quality Control: Full traceability from raw materials to final product.
Technical Support: Fast response from in-house chemists for COA, sample requests, or regulatory inquiries.
One-Stop Sourcing: Extensive catalog of platinum group metal compounds and specialty chemicals.
Whether you're a pharmaceutical R&D center, materials lab, or chemical distributor, UIV Chem is committed to delivering reliable Diamminedinitritoplatinum solutions with speed, safety, and consistency. Contact us today for a quote, sample request, or technical data sheet.