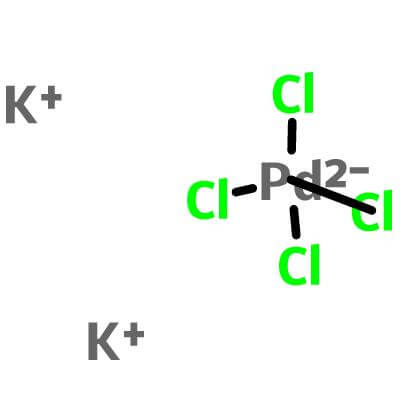
Potassium Tetrachloropalladate(II)
| Identification | ||
| Name |
|
Potassium Tetrachloropalladate(II) |
| Synonyms |
|
Potassium tetrachloroplatinate; Potassium chloropalladite dipotassium,tetrachloropalladium(2-); Potassium tetrachloroplatinate(II); Premion,Metals Basis); Potassium palladium(II) chloride Potassium tetrachloropalladate; |
| Molecular Formula |
|
K2PdCl4 |
| Molecular Weight |
|
326.41 |
| CAS Registry Number |
|
10025-98-6 |
| EINECS |
|
233-049-3 |
| Pd Content |
|
32.50% |
| Properties | ||
| Density |
|
2.67 g/mL at 25 °C(lit.) |
| Melting point |
|
105 °C (dec.)(lit.) |
| Solubility |
|
Soluble in water |
| Appearance |
|
Reddish brown crystalline solid |
| Safety Data | ||
| Hazard Symbols |
|
Xi |
| Risk Codes |
|
R36/38 |
| Safety Description |
|
S26;S37/39 |
Potassium Tetrachloropalladate(II) uses:
Potassium tetrachloropalladate(II) is widely used as a reagent and as a source of palladium chloride to make other palladium complexes and palladium nanoparticles. It finds an application in the synthesis of semiconducting metal-containing polymers. It also reacts with bis(dithiolates) to form metal-bis(dithiolates), which is used in bar code material, laser Q-switch materials, optical CD recording media and superconductivity.