| Identification | ||
| Name |
|
1-Ethylcyclohexyl methacrylate |
|
Synonyms |
|
1-Ethylcyclohexylmethacrylate (1-ethylcyclohexyl)2-methylprop-2-enoate EtCHMA1-EthylcyclohexylmethacrylateArFmonomers EtCHMA2-Propenoicacid,2-methyl-,1-ethylcyclohexylester 2-Propenoicacid,2-methyl-,1-ethylcyclohexylester MethacrylicAcid1-EthylcyclohexylEster |
|
|
||
| Molecular Structure |
|
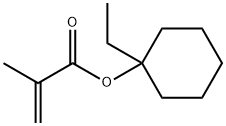
|
|
|
||
| Molecular Formula |
|
C12H20O2 |
| Molecular Weight |
|
196.29 |
| CAS Registry Number |
|
274248-09-8 |
| Properties | ||
| Solubility |
|
Very slightly soluble |
| Density |
|
0.94±0.1 g/cm3 |
| Boiling point |
|
247.3±9.0 ºC |
| Flash point |
|
96.0±16.1 ºC |
1-Ethylcyclohexyl Methacrylate (stabilized with MEHQ) is a monomer used in the preparative synthesis of biomedical polymers. It contributes to drug delivery systems, effectively carrying specific medications to targeted cells or organs in the body.
Properties:
1-Ethylcyclohexyl methacrylate has a low vapor pressure and a high flash point. It is miscible with many organic solvents but insoluble in water. This compound exhibits good light resistance and chemical corrosion resistance.
Applications:
1-Ethylcyclohexyl methacrylate is an important monomer widely used in coatings, inks, adhesives, and the plastics industry. It serves as a precursor for polymer synthesis and is also used in the production of polymethacrylate copolymers to enhance flexibility and chemical resistance.
Synthesis:
1-Ethylcyclohexyl methacrylate is typically synthesized by the reaction of methacrylic acid ester with 1-ethylcyclohexanol. The reaction requires a catalyst and solvent, with controlled temperature and reaction time.
Safety Information:
1-Ethylcyclohexyl methacrylate may cause irritation to the eyes and skin. Appropriate protective measures should be taken to avoid direct contact during handling.
Q. What are the optimized synthesis protocols for 1-ethylcyclohexyl methacrylate, and how can reaction yields be improved?
- Methodological Answer : The synthesis involves reacting methacrylic acid (0.5 mol) with 1-ethylcyclohexene (1.0 mol) in toluene using sulfuric acid (5 mmol) as a catalyst under nitrogen atmosphere . Key parameters include:
- Molar ratio : Excess 1-ethylcyclohexene (2:1 molar ratio to methacrylic acid) improves esterification efficiency.
- Catalyst selection : Acidic catalysts like H₂SO₄ enhance reaction rates but require careful control to avoid side reactions.
- Purification : Column chromatography and NMR/MS analysis are critical for isolating the product (69.9% yield) .
- Table :
| Parameter | Optimal Condition |
|---|---|
| Solvent | Toluene |
| Catalyst | H₂SO₄ (1 wt%) |
| Reaction Temperature | 80–100°C (reflux conditions) |
Q. Which analytical techniques are most reliable for confirming the structural integrity and purity of this compound?
- Methodological Answer :
- ¹H NMR : Peaks at δ 6.12 (1H, s) and δ 5.55 (1H, s) confirm the methacrylate group, while δ 0.89 (3H, t) corresponds to the ethyl group .
- Mass Spectrometry : Molecular ion peak at m/z 288 (M⁺) .
- Chromatography : Column chromatography (silica gel, hexane/ethyl acetate) removes unreacted precursors.
Q. What are the key considerations for stabilizing this compound during storage in laboratory settings?
- Methodological Answer :
- Inhibitors : Use hydroquinone monomethyl ether (MEHQ, 50–100 ppm) to prevent radical polymerization .
- Storage : Keep at 2–8°C under inert gas (N₂/Ar) to minimize moisture and oxygen exposure.
Q. How does the steric and electronic environment influence the reactivity of this compound in radical polymerization processes?
- Methodological Answer :
- The bulky 1-ethylcyclohexyl group introduces steric hindrance, slowing propagation rates compared to smaller methacrylates (e.g., methyl methacrylate) .
- Electronic effects : The electron-donating cyclohexyl group stabilizes the radical intermediate, favoring controlled polymerization.
- Experimental design : Compare kinetic data (e.g., kₚ values) with structurally analogous monomers using pulsed laser polymerization (PLP).
Q. What mechanistic insights explain the rearrangement of intermediates during the synthesis or derivatization of this compound?
- Methodological Answer :
- In low-nucleophilicity media (e.g., TFE or SbF₅-SO₂ClF), the cyclooctyl cation intermediate rearranges to the 1-ethylcyclohexyl cation due to steric strain relief and tertiary carbocation stabilization .
- Experimental validation : Use deuterium labeling or in-situ NMR to track skeletal rearrangements.
Q. What experimental strategies can be employed to assess the chronic toxicity of this compound in biomedical research applications?
- Methodological Answer :
- In vitro models : Use human dermal fibroblasts to evaluate cytotoxicity (IC₅₀) and oxidative stress markers (e.g., ROS levels) .
- In vivo studies : Administer subchronic doses (e.g., 50–200 mg/kg/day) to rodents over 90 days, monitoring neurological and hepatic endpoints .
- Analytical methods : LC-MS/MS for quantifying metabolic byproducts (e.g., methacrylic acid) in biological samples.